Describe the Net Rate of the Reaction at Equilibrium
Which one of the following statements does not describe the equilibrium stateA Equilibrium is dynamic and there is no net conversion to reactants and productsB The concentration of the reactants is equal to the concentration of the productsC The concentration of the reactants and products reach a constant levelD The rate. K C l D m A j B k K is the equilibrium constant.

8 2 Chemical Equilibrium Chemistry Libretexts
Apexvs answer 20 x 10-3.

. The rate of the second reaction its reverse is the product of the rate constant k -1 and the concentration of C andor D. Describe the equilibrium state of a reversible enzyme-catalyzed chemical reaction. The system is closed and all reactants and products are present.
All of the products are consumed. Q c is equal to K c. Chemical equilibrium is a state in which no net change in the proportions of reactants and products occurs during a reversible chemical reaction.
Add your answer and earn points. Which of the following statements describe a reaction that is at equilibrium. They arent really related but the rate of reaction for both sides of a reversible reaction is equal at equilibrium.
Which one of the following statements does not describe the equilibrium state. Increase in the reverse reaction rate. Cotton Activation Energy is being supplied Activated Complex 2.
Compare the rate of this reaction to the rate of the first reaction If the rate didnt change reaction is 0 order with respect to F2 If the rate doubled reaction is 1st order with respect to F2 If rate increased by a factor of four reaction is 2nd order with respect to F2 We identify the rate with respect to F2 with the symbol n The rate law will be. No net change is occurring. Are the molar concentrations of A B C D etc.
All enzyme molecules have substrates bound to their active sites. The value of Q c must increase in order for the reaction to reach equilibrium. Y C z D w A x B.
As a reaction is approaching equilibrium describe how the following change. For the chemical reaction. See the answer See the answer done loading.
Equilibrium of the reaction shift towards the right. Rate of reaction is quite simply how fast a reaction occurs Ie the time it takes for a set amount if product to be produced. The rate of the first reaction is the product of a rate constant k 1 and the concentration of A andor B.
Choose one or more. If this is true then the reaction is at equilibrium. This is due later today and I have no idea.
The rate of the forward reaction is equal to the rate of the reverse reaction. The reaction shown below reaches equilibrium with the concentrations CO2 equals 015 CO equals 003 O2 equals 005 What is the equilibrium constant for this reaction. For a chemical reaction the equilibrium constant can be defined as the ratio between the amount of reactant and the amount of product which is used to determine chemical behaviour.
Some of the SO 2 or O 2 to form SO 3. Eventually the rates of the forward and reverse reactions become equal. At the chemical equilibrium state the rate of forwarding reaction and reverse reactions becomes equal and the concentrations of products and reactants remain constant.
JA kB lC mD. Law of equilibrium - The principle that at chemical equilibrium in a reversible reaction the ratio of the rate of the forward reaction to the rate of the reverse reaction is a constant for that. The reverse reaction rate increases as equilibrium is approached because as the reaction goes from left to right the concentrations of the products increases therefore there are more product collisions causing the reverse reaction rate to increase.
Are coefficients in a balanced chemical equation. Describe the net rate of the reaction at equilibrium. High School Describe the net rate of the reaction at equilibrium.
The system contains too much. Rates of Reaction OBJECTIVES Identify four factors that influence the rate of a chemical reaction. Rate ClO2m F2n.
Increase in the forward reaction rate. Thus the reaction has to convert some of the reactants into products to come to equilibrium. The amount of reactants equals the amount of products.
Some of the SO 3 would change to SO 2 or O 2. All of the reactants are consumed. At equilibrium Rate of the forward reaction Rate of the backward reaction.
No further changes occur in the concentrations of reactants and products even though the two reactions continue at equal but opposite rates. In chemistry equilibrium describes a scenario where there is no net movement between reactants and products of a reaction. Describe the net rate of the reaction at equilibrium.
Louisabarth2 is waiting for your help. A Equilibrium is dynamic and there is no net conversion to reactants and products. At equilibrium the concentrations of reactants and products do not change.
The amount of reactants and. Add your answer and earn points. A B C D etc.
Q c is larger than K c. Think about the general reactions. B The rate of the forward reaction is equal to the rate of the reverse reaction.
R f r b Or kf α AaBb kb α Cc Dd. All reactants have been converted to products. If the product increases then.
W A x B y C z D. When a reaction takes place at the same rate as its reversible backwards reaction no net change is observed so the reaction is said to be at equilibrium Pearson 2014. Reaction Rates and Equilibrium Pre-AP Chemistry Charles Page High School Stephen L.
C The concentration of the reactants and products reach a constant level. The equilibrium expression is. Click here to get an answer to your question Describe the net rate of the reaction at equilibrium presleystevenss8743 presleystevenss8743 6 days ago Chemistry High School Describe the net rate of the reaction at equilibrium presleystevenss8743 is waiting for your help.
Describe the equilibrium state of a reversible enzyme-catalyzed chemical reaction. Rates of Reaction OBJECTIVES Describe how to express the rate of a chemical reaction. The system contains too much reactant and not enough product to be at equilibrium.
But the forward and reverse reactions have not stopped - they are still going on and at the same rate as each other. The forward and reverse reactions are proceeding at the same rate. J k l m etc.
New questions in Chemistry. Equilibrium of the reaction shift to decrease the concentration of the sulfur trioxide.
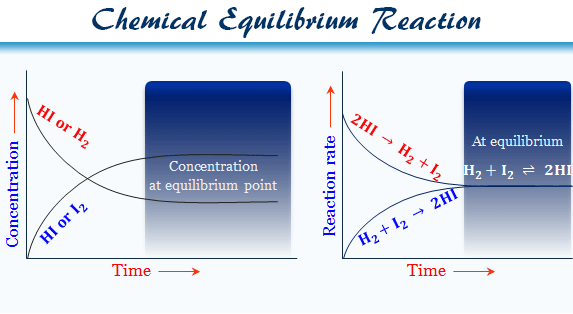
Chemical Equilibrium Reaction Definition Examples Types

0 Response to "Describe the Net Rate of the Reaction at Equilibrium"
Post a Comment